Metabolic Pathways
- Chemical changes in living organisms often happen with a number of stages. Each stage has its own specific enzyme.
- Catabolic pathways breakdown molecules.
- Anabolic pathways build up molecules.
- Enzyme 1 is specific only to substrate 1. It is then converted to product 1.
- Enzyme 2 is specific only to product 1 which becomes the substrate and then converted to product 2.
- Enzyme 3 is specific to product 2 which becomes the substrate and converted to product 3.
- Product is called the 'End product'.

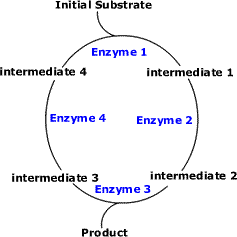
Cyclic Pathways (Example: Krebs Cycle and Calvin Cycle)
- The initial substrate is fed into the cycle.
- Enzyme 1 combines the regenerated 'intermediate 4' with the initial substrate to catalyses the production of intermediate 1.
- Enzyme 2 is specific to intermediate 1 and converts intermediate 1 to intermediate 2.
- Enzyme 3 is specific to intermediate 2 and catalyses it conversion to product and intermediate 3.
- Enzyme 4 is specific to intermediate 3 and catalyses its conversion to intermediate 4.
- The difference is the regeneration of the intermediate, in this case intermediate 4.
- Enzymes are very specific to certain substrates, like a lock is to a certain key.
- There is 1 enzyme for every substrate.
- The substrate induces change in a active site - the enzyme "adjusts" its structure to accommodate the substrate.
Activation Energies
 | ||
| Exergonic reactions |
- Enzymes lower the activation energy of the chemical reaction that they catalyse.
- In the activated complex or transition state energy is put into the substrate to make structure weak. This allows the reaction to occur with a minimal amount of additional energy required.
- Normal activation energy would cause damage to the proteins of the cell. So reduced activation energy make these reactions possible in a cell.
- After the product is formed, energy is released.
- Exergonic reactions release more energy than the activation energy.
- Inhibitors - substances that reduce or completely stop the action of an enzyme.
- Inhibition can act on the active site (competitive) or on another region of the enzyme molecule(non-competitive). The competition in the former being for the active site of the enzyme.

- The substrate and inhibitor are chemically very similar in molecular shape.
- The inhibitor can bind to the active site.
- Enzyme-inhibitor complexing blocks substrate from entering the active site. This blockage reduces the rate of reaction.
- If the substrate concentration is increased it occupies more active sites than the inhibitor. Therefore the substrate out-competes the inhibitor for the active site.
- The rate of reaction will increase again.
SDase can be inhibited by a later intermediate in the cycle called malonate.
- When a competitive inhibitor is present the rate of reaction is reduced.
- Increasing the concentration of the substrate reduces the effect of the inhibitor.
- At high concentrations the substrate out-competes the inhibitory molecules for the active site. The rate of reaction therefore increases.
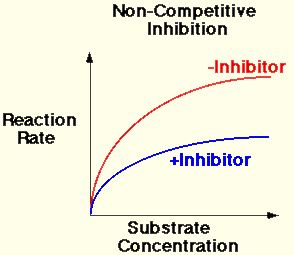
B. Non-competitive Inhibitors
- The substrate and the inhibitor are chemically different in molecular structure.
- The inhibitor cannot bind to the active site, but the inhibitor can bind to another region of the enzyme molecule.
- The bonding of the inhibitor with the enzyme causes structural changes in the enzyme molecule.
- The active site then changes shape.
- The substrate cannot bind therefore the rate of reaction decreases.
Silver ions inhibiting the formation of sulphide bridges at the amino acid cysteine.
This changes the protein bonding and in turn the active site changes excluding the substrate.
- The presence of an non-competitive inhibitor always significantly reduces the rate of reaction.
- Increasing the concentration of the enzyme increases the chance of a collision between the substrate and an enzyme that is not inhibited already. Therefore the rate can increase.
- The rate of reaction is always lower when the inhibitor is present.
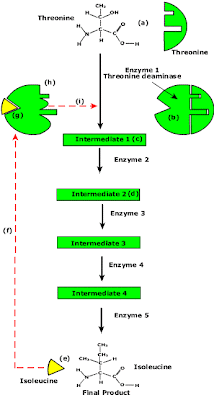
End Product Inhibition of Enzyme Pathway
- Enzyme pathways can be controlled by concentration of products from the end of the pathway.
- The principle is illustrated by the transamination (change R group) of the amino acid threonine to isoleucine.
- Isoleucine the end product, this molecule can inhibit the enzyme Threonine Deaminase.
- The inhibition occurs at an inhibition site on the enzyme but not the active site.
- An excess of end product (Isoleucine) switches off any more production of that product, isoleucine.
- At high concentrations, Isoleucine attaches to the inhibition site of Threonine deaminase.
- This attachment causes the active site of the enzyme to change blocking any further reaction.
- Isoleucine is used up in cellular processes that require this particular amino acid.
- The isoleucine concentration in the cell falls and so the Isoleucine that is attached to the enzyme detaches. This amino acid is also used up in the various cellular processes.
- With the inhibitor removed the the active site then becomes active again and the pathway switches back on.
- The isoleucine is again in production but once high concentrations are reached the pathways is once more inhibited. The process then cycles on in alternating stages of production and inhibition.
- Notice the similarity with non-competitive inhibition.
- This mechanism makes the pathway self-regulating in terms of product manufacture.




No comments:
Post a Comment