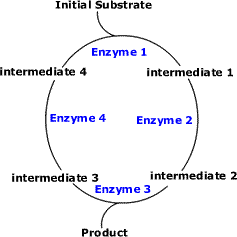
8.2.1. Chloroplast structure:
The chloroplast is a large complex double membrane organelle
that performs the function of photosynthesis within plant cells and also
contains a substance called chlorophyll that is essential for photosynthesis.
The chlorophyll in addition also produces a sugar from the sun this sugar is
made within the cell; this provides food for all the organelles.
Inter membrane:The
membrane of the mitochondria that is the site of electron transport and
chemiosmosis., where the electron transport chain occurs, Where the electron
transport chain of cellular respiration occurs
Outer membrane:Forms
a boundary between mitochondrion and cytoplasm; helps define the inner membrane
space, smooth membrane in mitochondria
Inner membrane space:Fluid
filled space between the inner and outer mitochondrial membranes, the region
between the inner membrane and the outer membrane of a mitochondrion or a
chloroplast. The main function of the intermembrane space is nucleotide
phosphorylation.
Stroma: Gel like
substance. Sugars are made in the stroma by the enzymes form the Calvin cycle.
Light independent reactions take place here.
Granum: A stacked
portion of the thylakoid membrane. The Grana functions in the light reactions
of photosynthesis
Thylakoid: The
disk shaped organelle. The structure of the thylakoid is the site of the
photosynthetic light reactions takes place. These are the reactions that
initially excite the electrons so they fall down the electron transport chain
and release ATP and continue on to release NADPH.
Lumen: this is the thylakoid membrane bound to
the chloroplast compartment.
Lamella: linking a thylakoid within one granum to
one in another. They are the sites of photosystem I. Simply put, lamellae may
be considered as a pair of membranes containing chlorophyll. It is placed
between the two primary cell walls of two plant cells and made up of
intracellular matrix.
8.2.2 STAGES OF PHOTOSYNTHESIS:
8.2.3 LIGHT DEPENDENT
REACTIONS: There are two
types of photosystems, Photosystem II and Photosystem I. When a chlorophyll
molecule absorbs light, the energy from this light raises an electron within
the chlorophyll molecule to a higher energy state. The chlorophyll molecule is
then said to be photo activated. Excited electron anywhere within the
photosystem are then passed on from one chlorophyll molecule to the next until
they reach a special chlorophyll molecule at the reaction center of the
photosystem. This special chlorophyll molecule then passes on the excited
electron to a chain of electron carriers.
Starts within
Photosystem II. As this excited electron passes from one carrier to the next it
releases energy. This energy is used to pump protons (hydrogen ions) across the
thylakoid membrane and into the space within the thylakoids. Then this forms a
gradient. Protons travel across the membrane down the concentration gradient,
but to do so the must pass through ATP synthase. This is located in the
thylakoid membrane. The energy used to move protons down the concentration
gradient is used to synthesis ATP from ADP and inorganic phosphate. The
synthesis of ATP is called non-cyclic photophosphorylation. Photosystem I then
accepts the electrons from the chain of electron carriers. These electrons
replace electrons previously lost from Photosystem I. Photosystem I then
absorbs light and becomes photo activated. The electrons become excited again
as they are raised to a higher energy state. These excited electrons then pass
along a short chain of electron carriers and are eventually used to reduce
NADP+ in the stroma. NADP+ accepts two excited electrons from
the chain of carriers and one H+ ion from the stroma to form NADPH. In
addition to producing NADPH, the light dependent reactions also produce oxygen
as a waste product.
Summery:
·
Light energy is converted into chemical energy.
▪
Chlorophyll molecules are attached to the
thylakoid membranes.
▪
They are often associated with accessory
pigments and other proteins to form Photosystem.
▪
At the center of all photosystem are forms of chlorophyll
a each of which is specialized to absorb a particular wavelength of light.
▪
Electrons within the chlorophyll absorb the
energy from photons and this raises them to higher 'excited' states.
Excited electrons are more easily lost from the chlorophyll,
which is a form of oxidation
8.2.5 LIGHT INDEPNDENT REACTONS: occurring in the stroma of the
chloroplast and involve the conversion of carbon dioxide and other compounds
into glucose. The light-independent reactions can be split into three stages;
these are carbon fixation, the reduction reactions and finally
the regeneration of ribulose bisphosphate. Collectively these stages are
known as the Calvin Cycle.
First stage: carbon fixation- The
single carbon in carbon dioxide is first trapped by Ribulose bisphosphate (5C)
to form a 2 molecules of Glycerate-3-phosphate (GP). The product of the reactions is a 6-carbon intermediate,
which is unstable and immediately splits in half to form two molecules of 3-
phosphoglycerate
Second stage: reduction- each molecule of 3-phisphogycerate
recives an additional phosphate group from ATP, becoming 1,3
bisphosphoglycerate. NADPH reduce the carboxyl group. Which store potential
energy. G3P is a sugar- the same three-carbon sugar formed by glycolysis. For
every three molecules of carbon dioxide there are six molecules of G3P. But
only one molecule of this three- carbon sugar can be counted as a net gain of
carbohydrate. One molecule exits
the cycle to be used in the plant.
Third stage: regeneration- In a complex series of reactions, the five
molecules of G3P are rearranged by the last steps of the Calvin cycle into
three molecules of RuBP. For this to happen uses three more molecules of ATP.
Now the RuBP is prepared to receive carbon dioxide again then the cycle happens
again.
-
The
regeneration of RuBP is essential for carbon fixation to continue. Five triose
phosphate molecules will undergo a series of reactions requiring energy from
ATP, to form three molecules of RuBP. RuBP is therefore consumed and produced
during the light-independent reactions and therefore these reactions form a cycle,
called the Calvin cycle.
8.2.4 PHOTOPHOSPHORYLATION
- As the electrons (released from
chlorophyll) cycle through the electron transport chains located on the
thylakoid membrane, they lose energy
- This free energy is used to pump H+ ions
from the stroma into the thylakoid
- The build up of protons inside the
thylakoid creates an electrochemical gradient (or proton motive force)
- The H+ ions return to
the stroma via the transmembrane enzyme ATP synthase, which uses the potential
energy from the proton motive force to convert ADP and an inorganic
phosphate (Pi) into ATP
- This process is called chemiosmosis
8.2.6 STRUCTURE AND FUNCTION OF THE
CHLOROPLAST
(for more detail refer to 8.2.1)
-Function- To capture light energy, which is stored
in ATP. This is all possible by the
A pigment called
chlorophyll; chlorophyll absorbs the energy from sunlight and utilize this
energy to synthesize food from carbon dioxide and water. Chloroplast is
involved in the photosynthesis process of the plants.
8.2.7 ACTION SPECTRUM AND ABSORPTION
SPECTRUM.
-Absorption spectrum: provides us clues to relative
effectiveness of different wavelengths for driving photosynthesis, since light
can perform work in chloroplast only if it is absorbed. (Wavelengths are absorbed and to what
extent.)
-The three curves show the wavelengths of
light best absorbed by three types of
pigments extracted from chloroplast.
-Action spectrum: Shows the relative performance of
different wavelengths. An action spectrum illuminates chloroplasts different
colors of lights and then plotting wavelengths against some measure of
photosynthetic rate such as carbon dioxide consumption or oxygen release. (Wavelengths of light can actually be
used to make photosynthesis work.)
-This graph plots the effectiveness of different wavelengths of light
during photosynthesis. The peaks of the action spectrum are broader than the
peaks in the absorption spectrum for chlorophyll and the valley is narrower and
not as deep.
8.2.8 LIMITING FACTORS ON THE RATE OF
PHOTOSYNTHESIS
-Light: Gradually the rate falls of and at a certain light intensity the rate
of photosynthesis stay constant.
-Temperature: The higher the temperature then the rate
of photosynthesis goes up. But when temperatures above 40°C the rate slows
down. This is because the enzymes involved in the chemical reactions of
photosynthesis are temperature sensitive and destroyed at higher temperatures.
-Carbon dioxide: The rate of photosynthesis increases linearly
with increasing carbon dioxide concentration. Gradually the rate falls at a
certain carbon dioxide concentration the rate of photosynthesis stays constant.